For the more than 100,000 women in the U.S. who undergo a mastectomy each year,1 removal of the breast to treat or prevent breast cancer can be lifesaving. The surgery is also life-changing—patients may experience anxiety and depression, studies show, as they adjust to a new body and self-image.2
That’s where reconstructive surgery, something roughly 40% of these women opt for post-mastectomy,3 can help. The operation can make women feel like themselves following a breast cancer diagnosis or treatment.4
“It’s a total quality-of-life decision,” says Mark Migliori, M.D., a plastic surgeon who performs breast reconstruction, of the choice to have the surgery. “We want to help the patient achieve a sense of wholeness.”
And yet, surgeons haven’t been able to offer this sense of wholeness to all patients, particularly those with large breasts or larger bodies. Dr. Migliori notes that this is because the largest-sized breast implants available are sometimes too small for these women.
As breast cancer rates5 continue to rise, more women will likely undergo mastectomies. The positive news? Some recent innovations are now helping surgeons meet the urgent need for more options for successful breast reconstruction surgery.
Setting the stage for optimal results
Under ideal circumstances, breast reconstruction will get patients to a place where they feel balanced and proportionate, and they don’t think about their breasts, Dr. Migliori explains. That usually starts with the mastectomy itself—it’s up to the oncologic surgeon to preserve the tissue, which will make the eventual reconstruction more successful.
In the U.S., the mastectomy is almost always performed by a surgeon other than the plastic surgeon, and collaboration and teamwork are critical to success. “I always say that the best reconstructions are the patients who have had the best mastectomies, meaning that the tissues have been respected and are healthy,” says Dr. Migliori.
Breast reconstruction can be done via breast implants or flap reconstruction. Flap procedures, also known as autologous reconstructions, involve using tissues from another part of the patient’s body, such as from their stomach, thighs or buttocks, to create a new breast.6 A flap procedure from the back (latissimus flap) is often used in conjunction with breast implants. In Dr. Migliori’s practice, breast implants are the most commonly used technique, with or without the use of a latissimus flap. Reconstruction can be initiated at the same time as a mastectomy, called an “immediate” reconstruction, or it can be initiated months or years later, which is known as a “delayed” reconstruction.7 Rarely is breast reconstruction completed in one step; it often requires multiple stages over the course of a year to optimize results.
Of course, women—and their breasts—come in all different shapes and sizes. Dr. Migliori notes surgeons have been traditionally limited in what they can offer individuals with larger frames or large breasts in particular, which comprise about 15% of post-mastectomy patients.8
The reason? The largest implants available are typically 800 cubic centimeters (cc), which can appear too small or have inadequate projection on larger bodies, Dr. Migliori says.
Previously, he notes, clinicians have been forced to make patients settle for smaller than their previous cup size. “We oftentimes couldn’t achieve a satisfactory reconstruction for them,” Dr. Migliori says. He adds that this can cause many patients to require more revision surgeries after their initial reconstruction.
Surgical complications can occur with any reconstruction, particularly with larger women. Some studies suggest that roughly 16% of implant-based reconstruction surgeries (and about 1.5% ofautologous reconstruction surgeries) fail for obese patients. Implants are still the preferred option for many patients and surgeons because the operation is usually shorter and less complicated than with autologous procedures.9 Many patients requiring this size reconstruction have other medical issues that make them ineligible for the autologous procedures.
A lack of sizing options means less-than-satisfactory outcomes
Johnson & Johnson MedTech recently developed two products to help close the gap: the MENTOR™ MemoryGel™ Enhance Breast Implant and the CPX™4 Plus Enhance Breast Tissue Expander. The silicone gel implants come in sizes 930 cc to 1445 cc, which is nearly twice as big as the previously largest size available. The implants were developed for use with the CPX 4 Plus Enhance Breast Tissue Expander, which is designed to create a pocket for the larger-volume implants.
Dr. Migliori was the first to use the larger-size implant in clinical trials starting in 2016, and he successfully performed the first surgery after FDA approval using the expander and larger implant in May 2025.
He and his team found that, following a mastectomy, skin tissues can be traumatized.10 This means that surgeons can’t put in a breast implant, especially one of a larger size, until the tissue has recovered. In this situation, the new expander is placed temporarily in the patient’s chest to create a pocket in which the implant will eventually go. It’s gradually filled with saline over a three-to-six-month period to slowly stretch the skin and help it recover.

The MENTOR™ MemoryGel™ Enhance Breast Implant
The expanders help illustrate to patients what the volume and placement of their breast implant will look like, Dr. Migliori says, which empowers them to ask for adjustments during the process. Once the expander reaches a patient’s desired size, it’s removed and the permanent breast implant is put in its place.
The two-stage reconstruction procedure allows surgeons to offer personalized results, Dr. Migliori says. “It’s a bit of an interactive process that allows the patient to get some sense of what the size might be that fits their proportion,” he explains. “It gives us the best chance for an informed decision by the patient.”
In clinical trials, the MENTOR MemoryGel Enhance Breast Implants received a 97% patient satisfaction rating. After three years, patients reported low complication rates and experienced improved physical, psychosocial and sexual well-being.11
According to Dr. Migliori, “having the ability to use an implant and extend the range of patients that we could successfully treat has been game-changing.”
These innovations give both surgeons and patients more options. Surgeons can help patients achieve their goals, and patients will no longer have to settle for reconstructive surgery that doesn’t truly meet their needs. Ultimately, Dr. Migliori says he hopes surgeons will be able to “extend our ability to achieve balance in a wider array of body types, empowering more women to consider breast reconstruction and improve their quality of life post-mastectomy.”
References:
- Mastectomy and double mastectomy. Mastectomy and Double Mastectomy - Brigham and Women’s Hospital. (n.d.). https://www.brighamandwomens.org/surgery/surgical-oncology/resources/mastectomy#:~:text=to%20Breast%20Surgery-,Mastectomy%20and%20Double%20Mastectomy,the%20risk%20of%20breast%20cancer.
- Luque Suárez, S., Olivares Crespo, M. E., Brenes Sánchez, J. M., & Herrera de la Muela, M. (2024). Immediate psychological implications of risk-reducing mastectomies in women with increased risk of breast cancer: A comparative study. Clinical Breast Cancer, 24(7), 620–629. https://doi.org/10.1016/j.clbc.2024.07.005
- Breast reconstruction after mastectomy | effective health care (EHC) program. (n.d.).https://effectivehealthcare.ahrq.gov/products/breast-reconstruction-mastectomy/protocol
- Roy, N., Downes, M. H., Ibelli, T., Amakiri, U. O., Li, T., Tebha, S. S., Balija, T. M., Schnur, J. B., Montgomery, G. H., & Henderson, P. W. (2024). The psychological impacts of post-mastectomy breast reconstruction: A systematic review. Annals of BreastSurgery, 8, 19–19. https://doi.org/10.21037/abs-23-33
- Breast cancer incidence still rises and death rate still declines. American Cancer Society. (2024, October 2). https://www.cancer.org/research/acs-research-news/breast-cancer-incidence-still-rises-and-death-rate-still-declines.html
- Friday, O. 13. (2023, October 13). Implant vs. flap breast reconstruction surgery: How to decide. Memorial Sloan Kettering Cancer Center. https://www.mskcc.org/news/breastreconstruction-deciding-between-implant-and-flap-surgery
- Kuhlefelt, C., Repo, J. P., Jahkola, T., Kauhanen, S., & Homsy, P. (2024). Immediate versus delayed breast reconstruction: Long-term follow-up on health-related quality of life and satisfaction with breasts. Journal of Plastic, Reconstructive & Aesthetic Surgery, 88, 478–486. https://doi.org/10.1016/j.bjps.2023.11.028
- Howarth, A. L., Rodriguez, A. M., Gargya, V., Lucas, H. D., & Mahabir, R. C. (2017). Larger breast implants warranted for post-mastectomy reconstruction. Plastic and Aesthetic Research, 4(12), 215. https://doi.org/10.20517/2347-9264.2017.80
- Cevallos, P., Berry, C., Lipman, K. J., Kubiak, C. A., Mohan, A. T., Ayyala, H. S., Manrique, O. J., & Nazerali, R. (2023). Breast reconstruction after mastectomy in patients with obesity: A narrative review. Annals of Translational Medicine, 11(12), 413413. https://doi.org/10.21037/atm-23-1599
- Robertson, S., Jeevaratnam, J., Agrawal, A., & Cutress, R. (2017). Mastectomy skin flap necrosis: Challenges and solutions. Breast Cancer: Targets and Therapy, Volume 9, 141152. https://doi.org/10.2147/bctt.s81712
- Alderman, A., Caplin, D., Hammond, D. C., Keane, A., Turetzky, J., & Kane, W. J. (2023). Clinical results of mentor Memorygel Xtra breast implants from the glow clinical trial. Aesthetic Surgery Journal, 43(12). https://doi.org/10.1093/asj/sjad272
Dr. Migliori is a consultant for Mentor Worldwide LLC but was not compensated for this article or his opinions in it.
The sale and distribution of MENTOR Breast Implant Devices are restricted to users and/or user facilities that provide information to patients about the risks and benefits of the device prior to its use in the form and manner specified in approved labeling to be provided by Mentor Worldwide LLC.
Important information: Prior to use, refer to the instructions for use supplied with this device for indications, contraindications, side effects, warnings and precautions.
Caution: US law restricts this device to sale by or on the order of a physician.
Important Safety Information:
The MENTOR™ Collection of Breast Implants are indicated for breast reconstruction.
Breast implant surgery should not be performed in women:
· With active infection anywhere in their body
· With existing cancer or pre-cancer of their breasts who have not received adequate treatment for those conditions
· Who are currently pregnant or nursing
Safety and effectiveness have not been established in patients with autoimmune diseases (for example lupus and scleroderma), a weakened immune system, conditions that interfere with wound healing and blood clotting, or reduced blood supply to breast tissue. Patients with a diagnosis of depression, or other mental health disorders, should wait until resolution or stabilization of these conditions prior to undergoing breast implantation surgery.
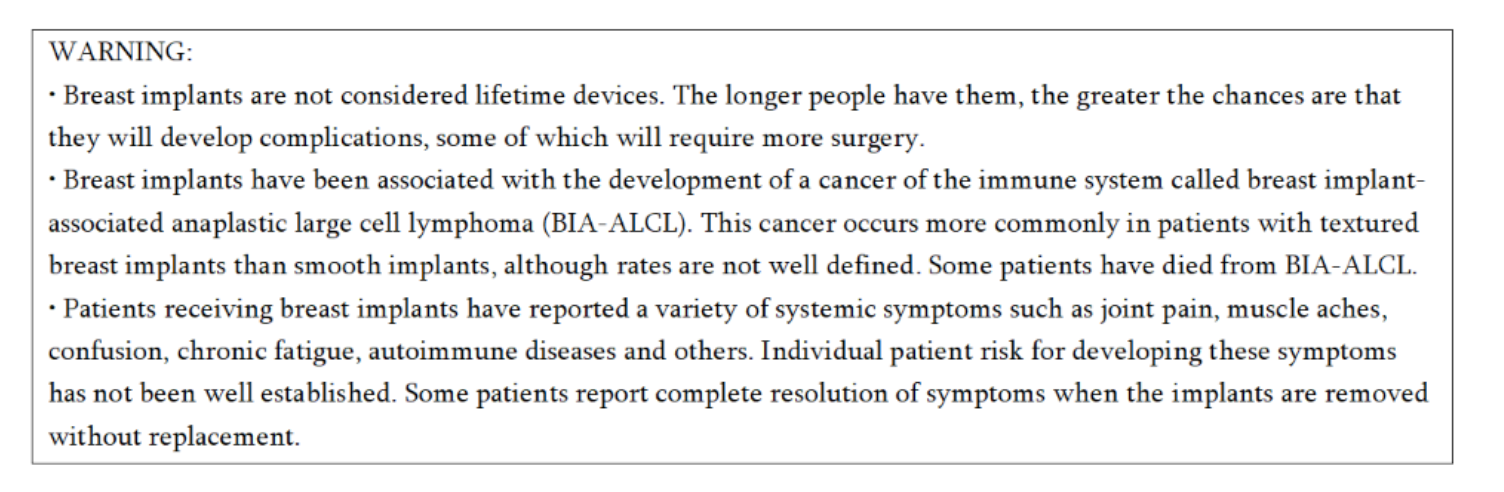
Breast implants are not lifetime devices and breast implantation may not be a one-time surgery. There are risks associated with breast implant surgery. The chance of developing complications increases over time. You may need additional unplanned surgeries on your breasts because of complications or unacceptable cosmetic outcomes. Many of the changes to your breast following implantation are irreversible (cannot be undone) and breast implants may affect your ability to breastfeed, either by reducing or eliminating milk production.
The most common complications for breast reconstruction with MENTOR™ MemoryGel™ Breast Implants include any reoperation, implant removal with or without replacement, and capsular contracture. The most common complications with MENTOR™ MemoryShape™ Breast Implants for breast reconstruction include reoperation for any reason, implant removal with or without replacement, and capsular contracture. A lower risk of complication is rupture. The health consequences of a ruptured silicone gel breast implant have not been fully established. MRI screenings are recommended three years after initial implant surgery and then every two years after to detect silent rupture. Breast implants are also associated with the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), an uncommon type of lymphoma. An individual's risk of developing BIA-ALCL with MENTOR™ Breast Implants is low based on the incidence of worldwide cases. The most common complications for breast reconstruction with MENTOR™ Saline-filled Breast Implants include re-operation, implant removal, capsular contracture, breast pain, and implant deflation.
Detailed information regarding the risks and benefits associated with MENTOR™ Breast Implants is provided in several educational brochures. For MemoryGel™ Implants: Important Information for Reconstruction Patients about MENTOR™ MemoryGel™ Breast Implants. For MemoryShape™ Implants: Patient Educational Brochure – Breast Reconstruction with MENTOR™ MemoryShape™ Breast Implants and Quick Facts about Breast Augmentation & Reconstruction with MENTOR™ MemoryShape™ Breast Implants. For MENTOR™ Saline-filled Implants: Saline-Filled Breast Implants: Making an Informed Decision. These brochures are available from your surgeon or visit www.mentorwwllc.com. It is important that you read and understand these brochures when considering MENTOR™ Breast Implants.
ARTOURA™ Breast Tissue Expanders and CONTOUR PROFILE™ Breast Tissue Expanders are used for breast reconstruction after mastectomy, correction of an underdeveloped breast, scar revision, and tissue defect procedures. The expander is intended for temporary subcutaneous or submuscular implantation and is not intended for use beyond six months. ARTOURA™ Breast Tissue Expanders and CONTOUR PROFILE™ Tissue Expanders contain a magnet within the internal injection domes and are NOT MRI compatible. The device could be moved by the MRI causing pain or displacement, potentially resulting in a revision surgery. DO NOT use the ARTOURA™ Breast Tissue Expander and CONTOUR PROFILE™ Tissue Expander in patients that have a previously implanted device such as pacemakers, drug infusion devices, artificial sensing devices, etc. that could be affected by a magnetic field. Mentor has not tested the effects of radiation therapy with ARTOURA™ Breast Tissue Expanders and CONTOUR PROFILE™ Expander devices. The incidence of extrusion of the expander has been shown to increase when the expander has been placed in injured areas: scarred, heavily irradiated or burned tissue, crushed bone areas, where severe surgical reduction of the area has previously been performed; and where steroids are used in the surgical pocket. Detailed information about indications, contraindications, warnings, and precautions associated with the use of ARTOURA™ Breast Tissue Expanders CONTOUR PROFILE™ Expanders are provided in the Instructions for Use (IFU) available online at www.mentorwwllc.com"
© Johnson & Johnson and its affiliates 2025. US_MNT_BRST_407201
