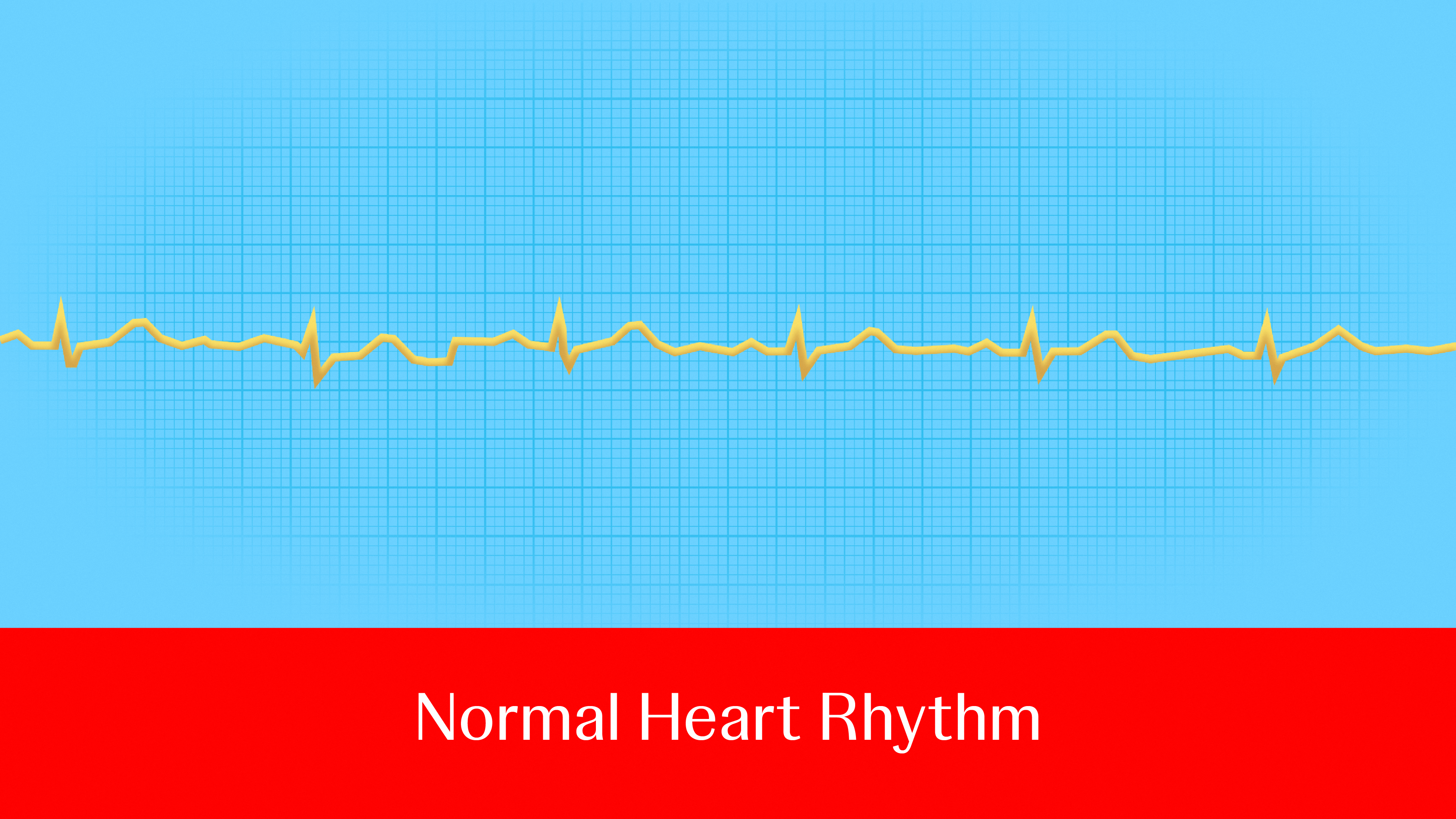
It’s been called “the 21st century cardiovascular disease epidemic,” affecting millions every year and raising the odds of serious health risks.
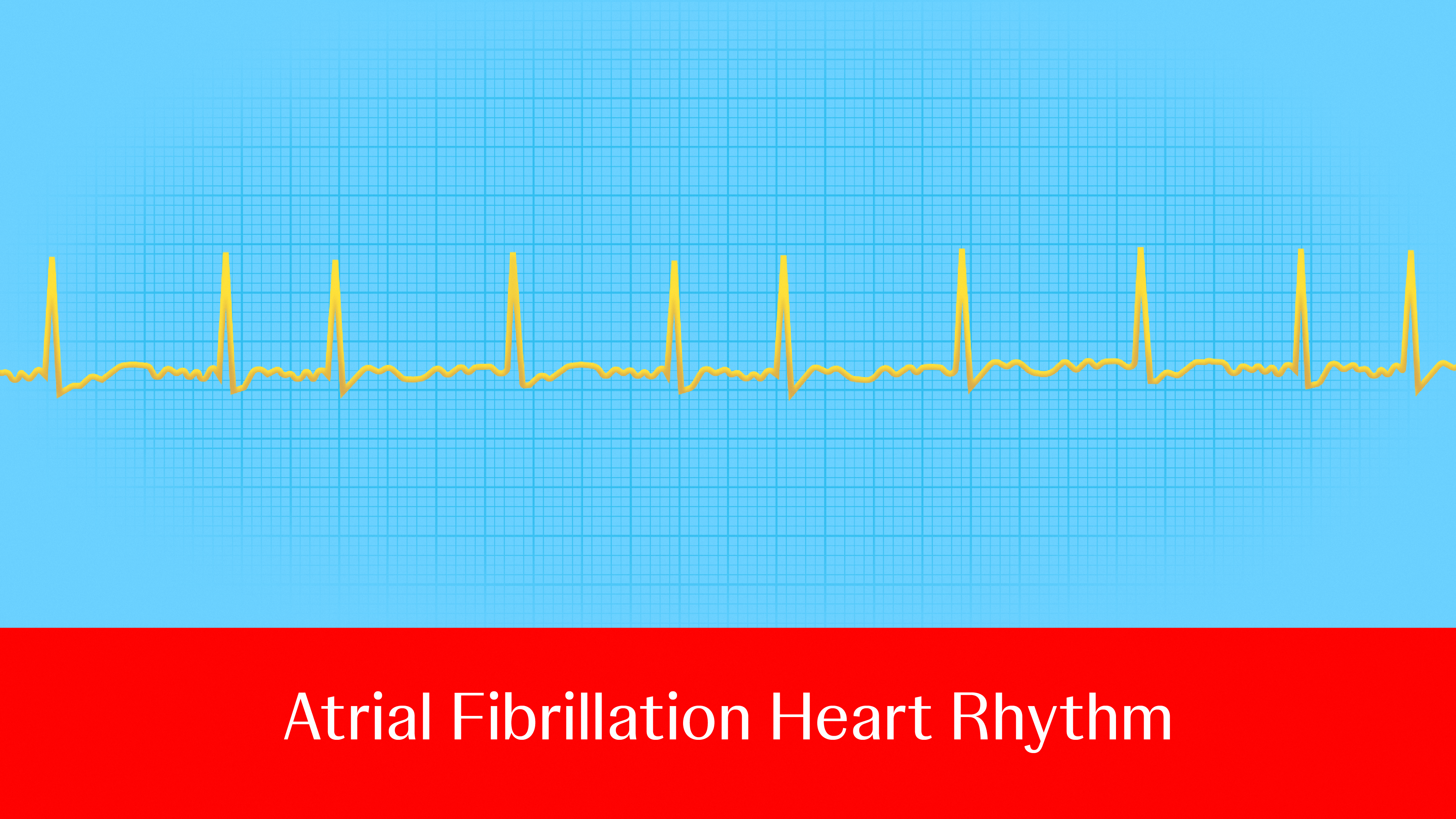
Atrial fibrillation (AFib), the most common type of heart rhythm disorder,1 is a major cause of stroke, heart failure, heart attack, dementia and death.2 About 1 in 3 to 5 people over the age of 45 will develop the condition,3 and it’s only becoming more prevalent: Between 2010 and 2019, cases rose from 33.5 million to 59 million globally—and these figures likely underestimate the impact, as many people don’t know they have AFib until they experience symptoms or a stroke. 4 What’s more, the number of people affected by AFib could increase by more than 60% by 2050, highlighting the critical need for effective treatment options.5
Ablation, one such option, restores the heart’s regular rhythm, reduces the occurrence of AFib episodes and relieves symptoms by creating scars in the heart to block faulty heart signals and restore a typical heartbeat.6
According to Philipp Sommer, Director of the Clinic for Electrophysiology and Professor of Cardiology at the Heart and Diabetes Center North Rhine-Westphalia University of Bochum, Bad Oeynhausen in Germany, the most common indication for ablation is symptom relief for patients with frequent, highly symptomatic AFib episodes.
Ablation is also a treatment option for patients with a risk of reduced heart function. Irrespective of symptoms in these patients, several studies have demonstrated that performing ablation to eliminate or reduce AFib can improve the heart’s ability to pump blood—known as ejection fraction. This can improve patient outcomes and extend life expectancy, shifting the procedure's benefit from just treating symptoms to improving prognostic factors.
With a relatively new type of ablation emerging as an effective option, here's a look at when and how different treatments may be considered.
The evolving ablation treatment landscape
Radiofrequency ablation (RFA) is a traditional (cardiac ablation was introduced around 30 years ago) and widely used method that uses radio waves to generate heat and disrupt the abnormal electrical signals that lead to rapid and irregular heartbeats. It’s primarily indicated for treating patients with AFib symptoms unresponsive to medications.7
A more recent development, pulsed field ablation (PFA), became commercially available in Europe in 2021 and in the United States in 2023.8 This treatment sends out quick, powerful bursts of electrical pulses to the target tissue in the heart; these pulses create nanopores in the cells which then compromise the integrity of the cell wall and eventually lead to cell death.9
With both RFA and PFA technology, mapping integration is critically important for electrophysiologists to ‘see’ inside the heart, which allows them to confidently deliver energy with precision. Integration with 3D mapping can provide lesion indexing and electrode tissue proximity indication give electrophysiologists real-time feedback that has proven to be critical for lesion durability and long-term outcomes. These advancements have shaped radiofrequency workflows for many years and remain as important with pulsed field energy.
A key distinction between RFA and PFA is the use of thermal energy, which influences some associated complications. Prof. Sommer explains that while the heat that RFA uses can be effective, it can result in rare but potentially serious complications, such as injury to surrounding tissues like the esophagus, pulmonary veins and the phrenic nerve. This damage could result in problems like atrial-esophageal fistula, pulmonary vein stenosis, stroke and phrenic nerve palsy, he explains.
RFA changes the cellular structure of the tissue during the procedure, causing a reaction much like an explosion on the heart's inner surface, Prof. Sommer says. This process could lead to inflammation, edema, and other reactions from the tissue responding to the burn. Patients often report chest pain—not just immediately, but up to two to four hours after the ablation procedure.
Pulsed-field ablation, on the other hand, avoids potentially affecting the surrounding healthy tissues by using rapid electric pulses to create holes that destroy target cells. It may also offer surrounding tissue protection, with lower rates of complications and (peri) esophageal injury.
With PFA, “the tissue will more or less look the same, except that the problematic cells are destroyed,” Prof. Sommer explains. Patients typically experience minimal pain after the procedure and can be discharged the same day without pain relief medications.
This said, both methods have a place in the treatment of AFib.
“Deciding which ablation method to use depends on the patient's clinical presentation and type of arrhythmia”
Prof. Sommer emphasizes that the safety of these methods depends heavily on how well their devices are programmed—in other words, he says, PFA may not be protective if used with the wrong tool or in improper settings. To address this, Prof. Sommer stresses the importance of a thorough testing and evaluation process to ensure that ablation procedures are performed safely and responsibly.
Importantly, comparing RFA and PFA is difficult because they don’t have the same volume of data from long-term studies. Decades of real-world evidence support RFA’s efficacy and safety. If patients ask about acute and long-term complications associated with RFA, Prof. Sommers says he can confidently report that complications are low based on the available evidence.
He also points out that scientists have analyzed more than 17,000 cases of PFA procedures worldwide and found no significant issues, such as pulmonary vein stenosis, and he says the evidence strongly supports the suggestion that PFA is also a safe and effective energy source.
When selecting an ablation method for a new AFib patient, the primary goal is typically to achieve solid pulmonary vein isolation or stop abnormal electrical signals in the heart, explains Prof. Sommer.10 In those cases, he considers PFA to be the safer and more effective option, which a 2024 study published in Nature Medicine supports.
However, he suggests that radiofrequency technologies are a better option in more challenging cases requiring an individualized and demanding approach, such as those involving complex anatomies, reoperations, and atrial tachycardias.
That said, with advancements coming in the near future, PFA could soon become just as versatile as RFA.
Ultimately, deciding which ablation method to use depends on the patient's clinical presentation and type of arrhythmia. Cost considerations are also important, as PFA technologies are usually more expensive than comparable radiofrequency tools.
Looking ahead to the future of ablation treatment, Prof. Sommer envisions the development of a device that would create high-density maps of the atria. This mapping would help doctors determine the most suitable ablation strategy for each patient. Ideally, the catheter used for this mapping could also perform the ablation procedure. Trials for these technologies are currently underway.
Another advance that’s being studied: technologies that could potentially deliver both radiofrequency and pulsed-field ablation—creating the potential for more options for patient and doctors alike.
Professor Philipp Sommer was compensated for his participation in this article, and any stated opinions and findings are based on his own knowledge and experience.
© Johnson & Johnson and its affiliates 2025 US_BWI_BTAD_394168
References:
1 Nesheiwat Z, Goyal A, Jagtap M. Atrial Fibrillation. [Updated 2023 Apr 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526072/
2 Becher N, Metzner A, Toennis T, Kirchhof P, Schnabel RB. Atrial fibrillation burden: a new outcome predictor and therapeutic target. Eur Heart J. 2024;45(31):2824-2838. doi:10.1093/eurheartj/ehae373
3 Linz D, Gawalko M, Betz K, et al. Atrial fibrillation: epidemiology, screening and digital health. Lancet Reg Health Eur. 2024;37:100786. Published 2024 Feb 1. doi:10.1016/j.lanepe.2023.100786
4 Linz D, Gawalko M, Betz K, et al. Atrial fibrillation: epidemiology, screening and digital health. Lancet Reg Health Eur. 2024;37:100786. Published 2024 Feb 1. doi:10.1016/j.lanepe.2023.100786
5 Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge [published correction appears in Int J Stroke. 2020 Dec;15(9):NP11-NP12. doi: 10.1177/1747493020905964]. Int J Stroke. 2021;16(2):217-221. doi:10.1177/1747493019897870
6 https://www.mayoclinic.org/tests-procedures/cardiac-ablation/about/pac-20384993
7 Verma A, Haines DE, Boersma LV, et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation. 2023;147(19):1422-1432. doi:10.1161/CIRCULATIONAHA.123.063988
8 Maurhofer J, Kueffer T, Madaffari A, et al. Pulsed-field vs. cryoballoon vs. radiofrequency ablation: a propensity score matched comparison of one-year outcomes after pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2024;67(2):389-397. doi:10.1007/s10840-023-01651-4 9 Ekanem E, Neuzil P, Reichlin T, et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat Med. 2024;30(7):2020-2029. doi:10.1038/s41591-024-03114-3
10 University Hospital South Hampton. Pulmonary vein isolation (PVI) ablation. Accessed November 7, 2024
.jpg&w=3840&q=75)