
Arbrea Breast Simulator Surgeon App for Mentor
A fast, app-based 3D simulator designed to drive more leads, increase conversion, and differentiate your practice.
Enhance your consultations with realistic 3D visuals, helping patients make decisions quickly — all without adding complexity to your workflow.
Please note this is a paid service.
Speak to a Rep or Book a Live Demo†
87%
Conversion rate
Practices using the Arbrea Breast Simulator have seen conversion rates as high as 87%1
3.4/4
Patient Match Result Score
Patients rated the similarity between their Arbrea Breast Simulator and actual surgical outcome at 3.4/4.2*
60s
Seconds for Capturing & Simulation2
Generate patient-specific 3D breast augmentation simulations in seconds with no external hardware required.
Create clarity and confidence in minutes.
The Arbrea Breast Simulator Surgeon App runs on iOS across tablet and phone devices, so you can align with your potential patient on size and profile at the point of discussion.
Speak to a Rep or Book a Live Demo†
In-Practice Surgeon Simulator
Drive higher conversion with fast, realistic 3D simulations created in seconds.
3D simulations generated in seconds
- No external hardware or internet connection needed
- Secure storage: images are never saved to the cloud
- Simplify patient decision-making with realistic visuals
Speak to a Rep or Book a Live Demo†


Practice-Branded Patient Simulator
Extend your digital presence and bring more qualified leads into your clinic.
- Optimize new leads with your own branded simulator
- Capture new patients with every download from your website
- Prepare patients before consultation to support better decision-making
Speak to a Rep or Book a Live Demo†
Frequently Asked Questions
How does the simulation work?
The app uses advanced 3D scanning and rendering to generate personalised breast augmentation simulations from standard Apple device cameras, no extra hardware required.
Does the app require Wi-Fi?
No. All simulations are generated on-device without internet connectivity. To get started, book a live demonstration here with our specialist team. Alternatively, you can get in contact with your local Mentor representative.
How is patient data stored?
Images are processed securely and never saved to the cloud. All simulations remain stored locally on your device.
How do I get started?
To get started, book a live demonstration here with our specialist team. Alternatively, you can get in contact with your local Mentor representative.
* Scale: 0 = Totally different, 1 = Moderately different, 2 = Similar, 3 = Very similar, 4 = Identical.
† We will use your information to register you to book a live demonstration, to provide technical support for our products and services and any other purpose based on your selection. You may withdraw your consent at any time. Please read our Privacy Policy.
1 Barbon C, Dibra E, Otte M. Pushing boundaries in aesthetic breast surgery: surgical planning based solely on online consultation and 3D simulation. Aesthetic Plast Surg. 2024;48(Suppl 1):S127. Abstract RF13.01. Presented at: ISAPS World Congress 2024; Cartagena, Colombia.
2 La Padula S, Pensato R, D’Andrea F, de Gregorio L, Errico C, Rega U, Canta L, Pizza C, Roccaro G, Billon R, et al. Assessment of Patient Satisfaction Using a New Augmented Reality Simulation Software for Breast Augmentation: A Prospective Study. J Clin Med. 2022;11(12):3464. doi:10.3390/jcm11123464
The third party trademarks used herein are the properties of their respective owners.
© Johnson & Johnson and its affiliates 2025. US_MNT_AMNT_410001
The sale and distribution of Mentor Breast Implant Devices are restricted to users and/or user facilities that provide information to patients about the risks and benefits of the device prior to its use in the form and manner specified in approved labeling to be provided by Mentor Worldwide LLC.
Important information: Prior to use, refer to the instructions for use supplied with this device for indications, contraindications, side effects, warnings and precautions.
Caution: US law restricts this device to sale by or on the order of a physician.
Important Safety Information:
MENTOR™ MemoryGel™ Breast Implants (excluding MemoryGel Enhance), MENTOR™ MemoryShape™ Breast Implants, and MENTOR™ Salinefilled Breast Implants are indicated for breast augmentation in women (at least 22 years old for MemoryGel™ Implants and MemoryShape™Implants, and 18 years old for Saline Implants) or for breast reconstruction (Including MemoryGel Enhance). Breast implant surgery should not be performed in women with active infection anywhere in their body, with existing cancer or pre-cancer of their breast who have not received adequate treatment for those conditions, or who are currently pregnant or nursing.
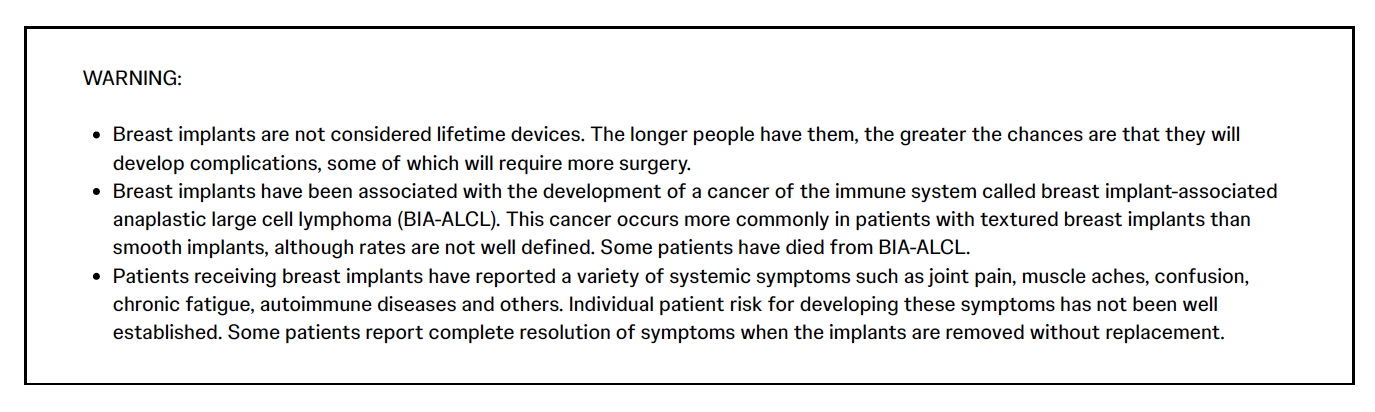
Breast implants are not lifetime devices and breast implantation may not be a one-time surgery. The chance of developing complications increases over time. The most common complications for breast augmentation and reconstruction with MemoryGel™ Implants include any reoperation, capsular contracture, and implant removal with or without replacement. The most common complications with MemoryShape™ Implants for breast augmentation include reoperation for any reason, implant removal with or without replacement, and ptosis. The most common complications with MemoryShape™ Implants for breast reconstruction include reoperation for any reason, implant removal with or without replacement, and capsular contracture. A lower risk of complication is rupture. The health consequences of a ruptured silicone gel breast implant have not been fully established. MRI screenings are recommended three years after initial implant surgery and then every two years after to detect silent rupture. Breast implants are also associated with the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), an uncommon type of lymphoma. An individual's risk of developing BIA-ALCL with MENTOR™ Breast Implants is low based on the incidence of worldwide cases. The most common complications with MENTOR™ Saline-filled Implants include reoperation, implant removal, capsular contracture, breast pain, and implant deflation.
For MemoryGel™ Implants, patients should receive a copy of Important Information for Augmentation Patients about MENTOR™ MemoryGel™ Breast Implants or Important Information for Reconstruction Patients about MENTOR™ MemoryGel™ Breast Implants. For MemoryGel™ Enhance Breast Implants: Important Information for Reconstruction Patients about MENTOR™ MemoryGel™ Enhance Breast Implants. For MemoryShape™ Implants, patients should receive a copy of Patient Educational Brochure – Breast Augmentation with MENTOR™ MemoryShape™ Breast Implants or Patient Educational Brochure – Breast Reconstruction with MENTOR™ MemoryShape™ Breast Implants, and a copy of Quick Facts about Breast Augmentation & Reconstruction with MENTOR™ MemoryShape™ Breast Implants. For MENTOR™ Salinefilled Implants, patients should receive a copy of Saline-Filled Breast Implants: Making an Informed Decision. Your patient needs to read and understand the information regarding the risks and benefits of breast implants, with an opportunity to consult with you prior to deciding on surgery.
The ARTOURA™ Breast Tissue Expander or CONTOUR PROFILE™ Breast Tissue Expander can be utilized for breast reconstruction after mastectomy, correction of an underdeveloped breast, scar revision, and tissue defect procedures. The expander is intended for temporary subcutaneous or submuscular implantation and is not intended for use beyond six months. ARTOURA™ Breast Tissue Expander and CONTOUR PROFILE™ Tissue Expanders include magnetic injection domes, which contain a rare earth permanent magnet, and are NOT MRI compatible. The device could be moved by the MRI causing pain or displacement, potentially resulting in a revision surgery. Do not use the ARTOURA™ Tissue Expander nor CONTOUR PROFILE™ Tissue Expander in patients where an MRI may be needed. DO NOT use the ARTOURA™ Breast Tissue Expander and CONTOUR PROFILE™ Tissue Expander in patients that have a previously implanted device such as pacemakers, drug infusion devices, artificial sensing devices, etc. that could be affected by a magnetic field. Mentor has not tested the in vivo effects of radiation therapy with ARTOURA™ Breast Tissue Expander and CONTOUR PROFILE™ Expander devices and cannot warrant the safety of such use. The incidence of extrusion of the expander has been shown to increase when the expander has been placed in injured areas: scarred, heavily irradiated or burned tissue, crushed bone areas, where severe surgical reduction of the area has previously been performed and where steroids are used in the surgical pocket.
For detailed indications, contraindications, warnings, and precautions associated with the use of all MENTOR™ Implantable Devices, which include MENTOR™ Saline-filled Implants, MemoryGel™ Implants, MemoryShape™ Implants, ARTOURA™ Expanders, and CONTOUR PROFILE™ Expanders, please refer to the Instructions for Use (IFU) provided with each product or visit www.mentorwwllc.com.